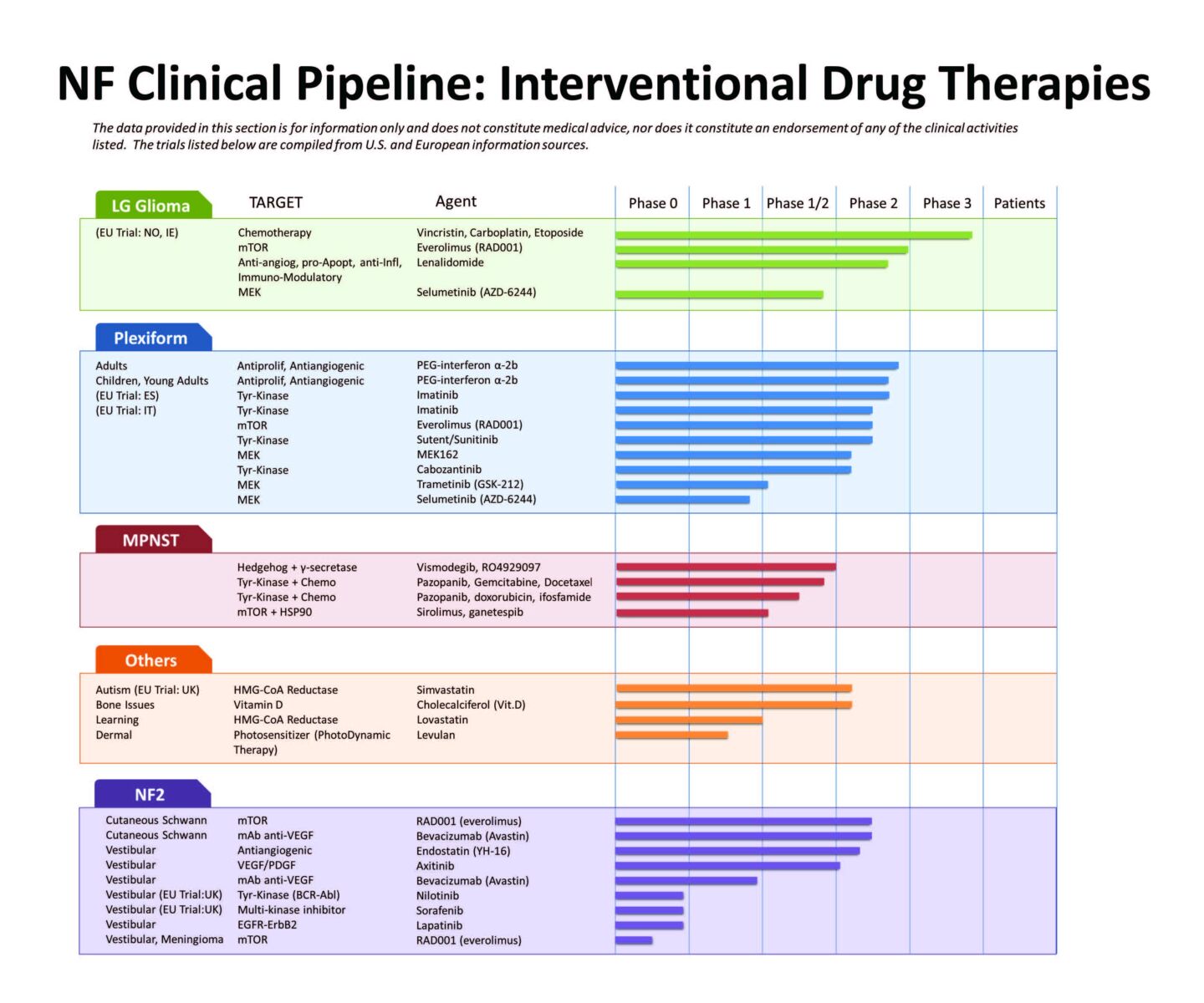
The Children’s Tumor Foundation has created chart shows all the drug treatments being tested for different conditions in NF1 and NF2 at the present time. This clinical drug pipeline includes completely new drugs, as well as variations of existing drugs, and new uses of existing drugs. Many of these potential treatments came out of research sponsored by the Children’s Tumor Foundation.
The chart below shows all the drug treatments being tested for different conditions in NF1 and NF2 at the present time. “Phase 0” is very early stage testing, “Phase 1” tests for drug safety, and “Phase 2” tests whether the treatment works. (Sometimes “Phase 1” and “Phase 2” are combined as “Phase1/2.”) “Phase 3” tests whether the new treatment is better than existing treatments.
This clinical pipeline includes completely new drugs, as well as variations of existing drugs, and new uses of existing drugs. Many of these potential treatments came out of research sponsored by the Children’s Tumor Foundation.
A few notes:
“Targets” are the part of the disease process that the drug tries to stop.
“Agents” are the drug names.
“Phases” are the current stage of research. The higher the number of the Phase, the closer the treatment is to being approved for use in NF patients.
To find out more about any of these clinical trials in the U.S., go to www.clinicaltrials.gov and search for the name of the drug, and NF. You can also find more information at www.nfregistry.org by clicking “Clinical Trials” on the menu bar. For clinical trials in Europe, search www.clinicaltrialsregister.eu.
Please click here to download an easier to read PDF of the NF clinical drug pipeline chart.