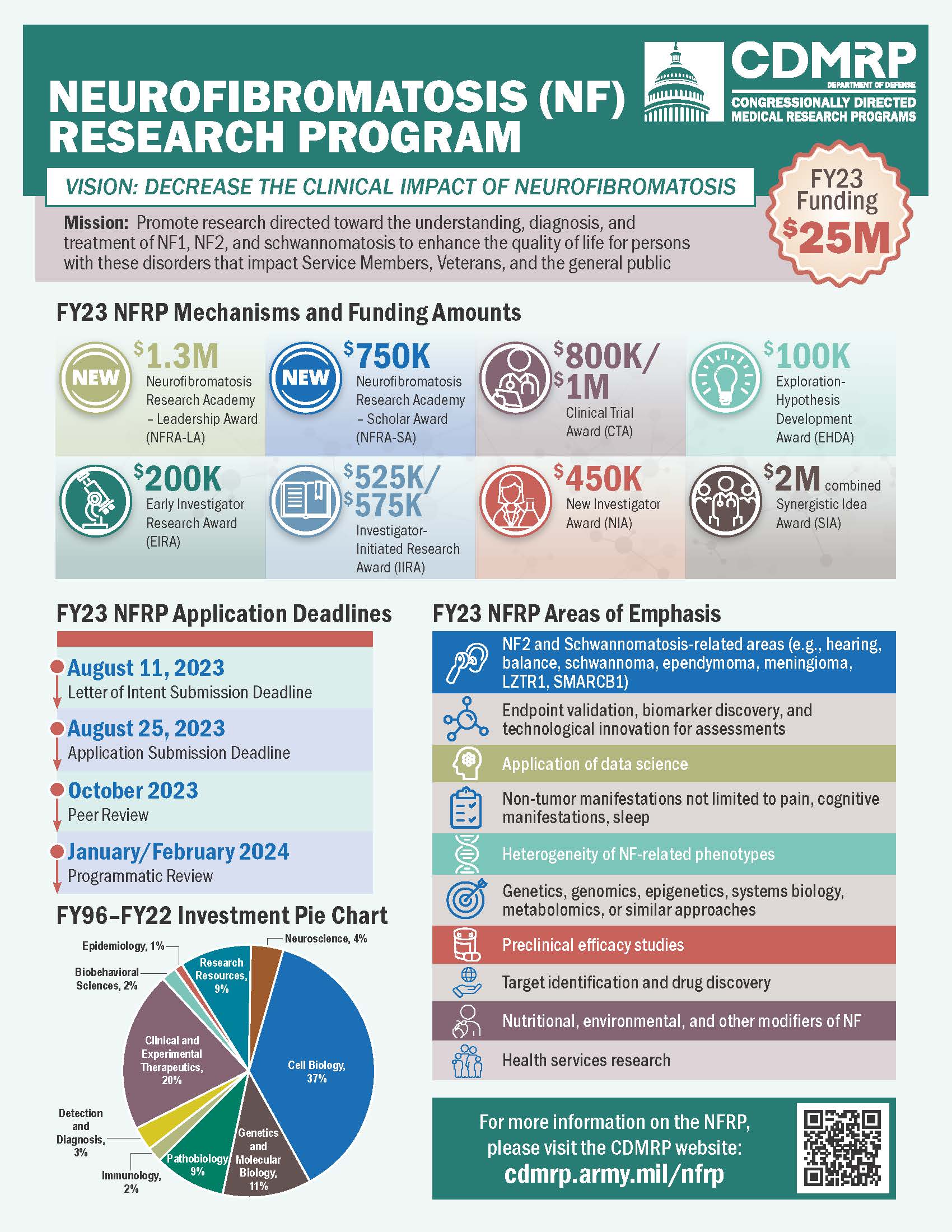
The FY23 Defense Appropriations Act provides funding for the Neurofibromatosis Research Program (NFRP) to support innovative, high-impact Neurofibromatosis (NF) research. The managing agent for the anticipated funding opportunities is the CDMRP at the U.S. Army Medical Research and Development Command (USAMRDC).
The FY23 NFRP funding opportunity announcements for the following award mechanisms will be posted on the Grants.gov website. Applications submitted to the FY23 NFRP must address one or more of the following Areas of Emphasis:
- NF Type 2 and Schwannomatosis-related areas (e.g., hearing, balance, schwannoma, ependymoma, meningioma, LZTR1, SMARCB1)
- Endpoint validation, biomarker discovery, and technological innovation for assessments
- Application of data science
- Non-tumor manifestations not limited to: pain, cognitive manifestations, sleep
- Heterogeneity of NF-related phenotypes
- Genetics, genomics, epigenetics, systems biology, metabolomics, or similar approaches
- Preclinical efficacy studies
- Target identification and drug discovery
- Nutritional, environmental, and other modifiers of NF
- Health services research
Neurofibromatosis Research Academy-Leadership Award (new for FY23) – Letter of Intent due August 11, 2023
Academy Director – Must be an independent and established Neurofibromatosis (NF) investigator at or above the level of Associate Professor (or equivalent) as of the full application submission deadline and be willing to commit at least 25% effort towards leading the Academy’s activities.
Deputy Director – Must be an independent NF researcher at the level of Associate Professor (or equivalent) as of the full application submission deadline and at a different institution from the Director. Must be willing to commit at least 25% effort towards leading the Academy’s activities.
Supports establishment and management of a multi-institutional interactive virtual research academy to foster collaborative research and career development.
- Vision, leadership/support, communications, and management plans must be clearly articulated.
- The academy will consist of Scholars (early-career investigators) and their Career Guides (primary mentors) from different institutions under the leadership of an Academy Director and Deputy Director
- Supports visionary individuals to serve in the Academy leadership (Director and Deputy Director). The Director and Deputy Director must have past NF1, NF2 and/or sch publication records in peer-reviewed journals and past and/or present NF1, NF2 and/or sch research funding.
- Clinical trials are not allowed.
- The maximum period of performance is 5 years.
- The maximum allowable funding for the entire period of performance is $1.3 million (M) in direct costs.
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
Neurofibromatosis Research Academy-Scholar Award (new for FY23) – Letter of Intent due August 11, 2023
Investigators – Must be within 7 years of their last terminal degree (Ph.D.) or clinical fellowship (M.D.) or equivalent as of the full application submission deadline and be willing to commit at least 50% protected time for NF research.
Career Guide (Mentor) – Independent and established NF investigators must be at or above the level of Associate Professor (or equivalent) and be willing to commit at least 5% effort towards mentoring activities.
- Health services research letter attesting to eligibility is required.
- Supports the addition of young investigators to a unique, interactive virtual academy that provides intensive mentoring to advance research in NF, national networking, and a peer group for junior faculty, including:
- Young, promising investigators who have innovative, high-impact ideas or new technologies with a clear path in the NF field.
- Early-career scientists or research clinicians (see eligibility) who are currently working in other areas and want to shift their focus to NF research.
- Early-career investigators whose ability to commit to conducting NF research is limited by lack of resources or other overwhelming obstacles are encouraged to apply.
- Requires a Career Guide (Mentor) who is an independent, established NF investigator with a record of NF publications in peer-reviewed journals. The Career Guide is not required to be at the same institution as the scholar; however, if at a different institution, a secondary Career Guide at the Scholar’s institution is needed.
- Career sustainment and development plans must be clearly articulated.
- Preliminary and/or published data are encouraged but not required.
- Clinical trials are not allowed.
- The maximum period of performance is 4 years.
- The maximum allowable funding for the entire period of performance is $750,000 in direct costs.
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
Clinical Trial Award – Letter of Intent due August 11, 2023
Investigators must be at or above the level of Assistant Professor (or equivalent).
- Supports research with the potential to have a major impact on the treatment or management of NF.
- Supports phase 0, 1, or 2 clinical trials relevant to NF and/or schwannomatosis; combinations of phases are permitted.
- Scientific rationale and preliminary data are required for phase 1 and 2 clinical trial applications.
- Must support a clinical trial and may not be used for preclinical studies.
- The maximum period of performance is 4 years.
- The maximum allowable funding for the entire period of performance is $800,000 in direct costs ($1.0M in direct costs for applications including a Collaborator).
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
Early Investigator Research Award – Letter of Intent due August 11, 2023
Investigators must:
- Be involved in a postdoctoral training or medical residency program;
- Possess up to 4 years of continuous postdoctoral research experience by application submission deadline; and
- Possess a doctoral degree (i.e., Ph.D., M.D./Ph.D., D.O./Ph.D.) or a clinical doctoral degree (i.e., M.D./D.O. or Ph.D. in a clinical discipline) from an accredited organization or program. Must commit at least 50% of effort to the project.
- Supports NF-focused research opportunities for individuals in the early stages of their careers.
- Investigators must have a designated mentor who is an experienced NF researcher.
- Clinical trials are not allowed.
- Eligible to participate in the Neurofibromatosis Research Academy.
- The maximum period of performance is 2 years.
- The maximum allowable funding for the entire period of performance is $200,000 in direct costs
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
Exploration – Hypothesis Development Award – Letter of Intent due August 11, 2023
Investigators may be from all academic levels (or equivalent).
- Supports the initial exploration of innovative, high-risk, high-gain, and potentially groundbreaking concepts in NF research.
- Preliminary and/or published data are encouraged but not required.
- Mentorship is highly encouraged.
- Clinical trials are not allowed
- The maximum period of performance is 2 years.
- The maximum allowable funding for the entire period of performance is $100,000 in direct costs.
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
Investigator-Initiated Research Award – Letter of Intent due August 11, 2023
Investigators must be at or above the level of Assistant Professor (or equivalent) and must plan to commit at least a 10% level of effort for each budget period throughout the entirety of the award.
- Supports highly rigorous, high-impact research projects that have the potential to make an important contribution to NF research and/or patient care.
- Optional Features: Applications meeting the criteria identified in the announcement may apply for a higher level of funding for the Qualified Collaborator or NF Open Science Initiative (NF-OSI) option.
- Preliminary and/or published data are required.
- Clinical trials are not allowed.
- The maximum period of performance is 3 years.
- The maximum allowable funding for the entire period of performance is $525,000 in direct costs.
- Maximum funding of $575,000 in direct costs for applications including either a Qualified Collaborator or NF-OSI Option.
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
New Investigator Award – Letter of Intent due August 11, 2023
Early-Stage Investigator (ESI) – An independent investigator who is at or below the level of Assistant Professor (or equivalent) and able to commit at least a 30% level of effort during each budget year toward the proposed NF research project.
Established Investigator (EI) –An independent investigator who is above the level of Assistant Professor and able to commit at least a 10% level of effort during each budget year toward the proposed NF research project
- ESI: Supports research conducted by promising independent investigators.
- EI: Supports the transition of established investigators who will transition from other research fields into a career in NF research.
- Prior experience in NF research is not required.
- Preliminary and/or published data are required.
- Clinical trials are not allowed.
- Must not have received a New Investigator Award previously from any program within the CDMRP.
- ESI: Eligible to participate in the Neurofibromatosis Research Academy.
- The maximum period of performance is 3 years.
- The maximum allowable funding for the entire period of performance is $450,000 in direct costs.
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
Synergistic Idea Award – Letter of Intent due August 11, 2023
Investigators must be at or above the level of Assistant Professor (or equivalent).
- Supports new or existing partnerships involving two or three principal investigators (PIs; Initiating PI and Partnering PI[s]) to address a central innovative question or problem in NF that may include high risk, provided there is a potential for significant impact.
- Application must demonstrate the synergistic components (i.e., leveraging disciplines, expertise, or critical resources) that will significantly advance the project such that the research outcomes as a whole will be realized rapidly and efficiently and could not otherwise be accomplished through the independent efforts of a single investigator.
- Preliminary and/or published data are required.
- Clinical trials are not allowed.
- The maximum period of performance is 3 years.
- The maximum allowable funding for the entire period of performance is $2.0M for direct costs (plus indirect costs).
- Indirect costs may be proposed in accordance with the institution’s negotiated rate agreement.
A pre-application is required and must be submitted through the electronic Biomedical Research Application Portal (eBRAP) prior to the pre-application deadline. All applications must conform to the final funding opportunity announcements that will be available for downloading from the Grants.gov website. The application package containing the required forms for each award mechanism will also be found on Grants.gov. A listing of all CDMRP and other USAMRDC extramural funding opportunities can be obtained on the Grants.gov website by performing a basic search using CFDA Number 12.420.
For email notification when announcements are released, subscribe to program-specific news and updates under “Email Subscriptions” on the eBRAP homepage. For more information about the NFRP or other CDMRP-administered programs, please visit the CDMRP website.
To download as a PDF, CLICK HERE.
Point of Contact:
CDMRP Help Desk
301-682-5507
help@eBrap.org

