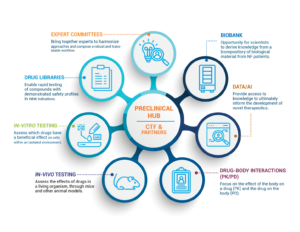
The Children’s Tumor Foundation is accelerating the path to drug discovery by constructing an NF-focused Preclinical Hub to supercharge the development of NF treatments. The Preclinical Hub is built on the successes of the Preclinical Consortium (2008-2016) and Synodos for NF2 initiatives (2014-2017), both of which efficiently delivered treatments to patients. Due to limitations in the scalability of both initiatives, CTF decided to expand the Preclinical Hub to become a full public-private partnership.
The Preclinical Hub will speed the approval of potential treatments by offering the following to academic, research, and pharmaceutical industry partners:
- Access to disease models, data tools, drug libraries, and biological material
- Expert advice and support during preclinical study design and execution
- Prenegotiated Master Service Agreements
- Predetermined protocols and tests
The Preclinical Hub is already underway and has announced a CTF-funding opportunity to support the generation of clinically relevant models for NF. Additionally, several case studies serve as examples of the function and power of this bold new initiative.
Case Studies
As part of the Preclinical Hub efforts, CTF was able to connect an AI-powered drug discovery company in the rare disease space with researchers holding key preclinical models for NF. Making this connection and sharing our prenegotiated workflows between the company and the expert research facility running the preclinical experiments is a great way to streamline drug development efforts. This will significantly shorten the timeline to the clinic.
We have also helped companies initiate an NF program and support early proof of concept studies that could ultimately lead to a clinical program for NF. In such cases, working through the Preclinical Hub has provided expert advice, connections to appropriate research experts running preclinical models, and resources for gathering the initial data necessary to potentially launch an NF program.
These examples are an early glimpse into what is possible with the Preclinical Hub initiative. We are delighted to share this progress as we continue to identify, validate, and share the most robust preclinical models for clinical translation, and accelerate the identification of clinical trial-ready therapeutics for NF.
To find out more, read out to Irene Morganstern at  imorganstern@ctf.org
imorganstern@ctf.org